
Classify each element in Conceptual Problem 1 ( Section 1.Other important groupings of elements in the periodic table are the main group elements, the transition metals, the lanthanides, and the actinides. Metals are lustrous, good conductors of electricity, and readily shaped (they are ductile and malleable), whereas solid nonmetals are generally brittle and poor electrical conductors. They are separated by a diagonal band of semimetals. Metals are located on the left of the periodic table, and nonmetals are located on the upper right. Semimetals exhibit properties intermediate between those of metals and nonmetals. The elements can be broadly divided into metals, nonmetals, and semimetals. Some of the groups have widely used common names, including the alkali metals (group 1) and the alkaline earth metals (group 2) on the far left, and the halogens (group 17) and the noble gases (group 18) on the far right. Elements that exhibit similar chemistry appear in vertical columns called groups (numbered 1–18 from left to right) the seven horizontal rows are called periods. The periodic table is an arrangement of the elements in order of increasing atomic number. As expected, semimetals exhibit properties intermediate between metals and nonmetals. Most solid nonmetals are brittle, so they break into small pieces when hit with a hammer or pulled into a wire. Nonmetals can be gases (such as chlorine), liquids (such as bromine), or solids (such as iodine) at room temperature and pressure. It is used as a nasal/sinus decongestant and stimulant, or as. Click on 'Development of the periodic table' to learn about the scientists involved in the table's creation. Pseudoephedrine is a sympathomimetic drug of the phenethylamine and amphetamine chemical classes. Nonmetals, in contrast, are generally poor conductors of heat and electricity and are not lustrous. Hover over an element to find out about its discovery and click on it for more information.
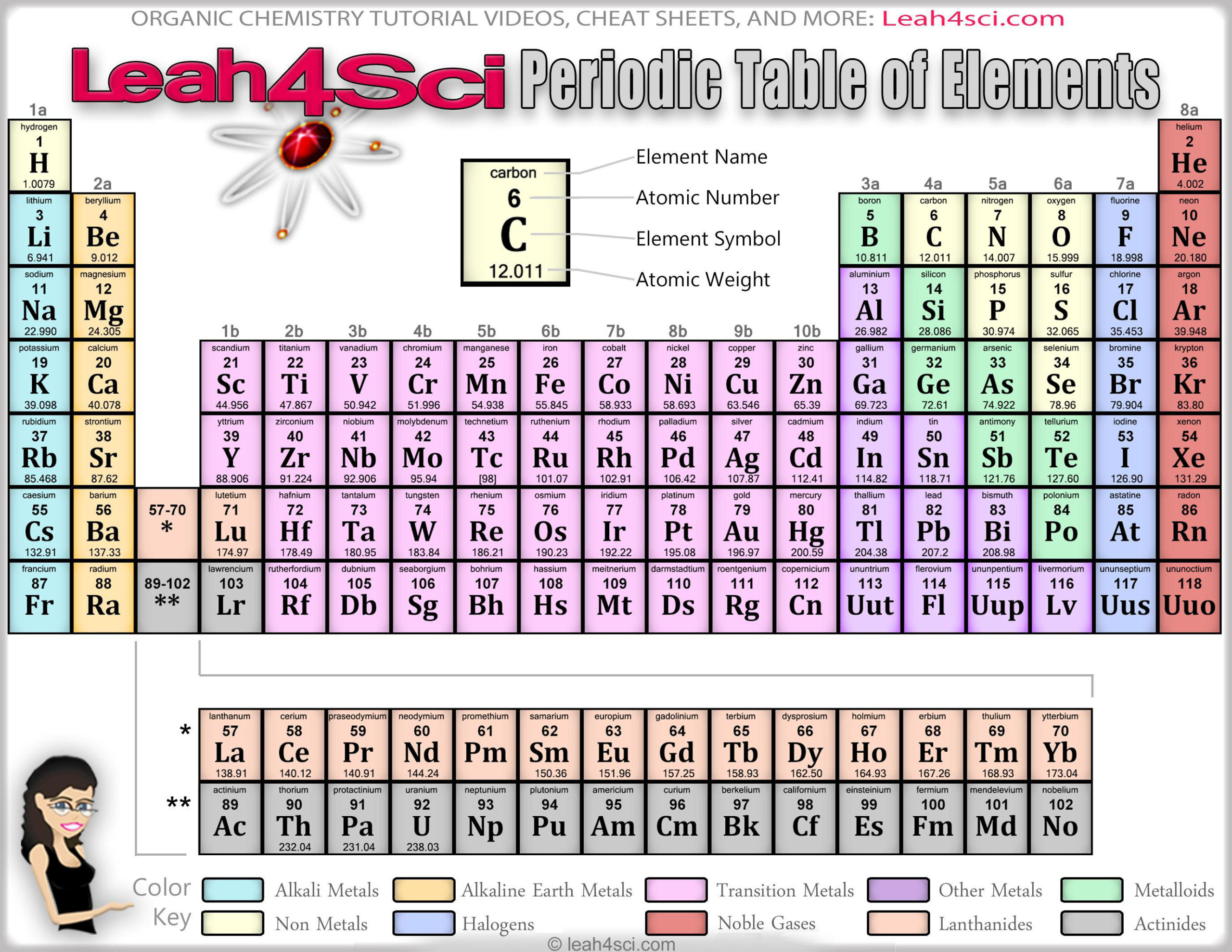
Of the metals, only mercury is a liquid at room temperature and pressure all the rest are solids. The vast majority of the known elements are metals. Metals-such as copper or gold-are good conductors of electricity and heat they can be pulled into wires because they are ductile they can be hammered or pressed into thin sheets or foils because they are malleable and most have a shiny appearance, so they are lustrous. The distinction between metals and nonmetals is one of the most fundamental in chemistry.

As you might expect, elements colored in gold that lie along the diagonal line exhibit properties intermediate between metals and nonmetals they are called semimetals. The heavy orange zigzag line running diagonally from the upper left to the lower right through groups 13–16 in divides the elements into metals (in blue, below and to the left of the line) and nonmetals (in bronze, above and to the right of the line). The semimetals lie along a diagonal line separating the metals and nonmetals. The metals are on the bottom left in the periodic table, and the nonmetals are at the top right.
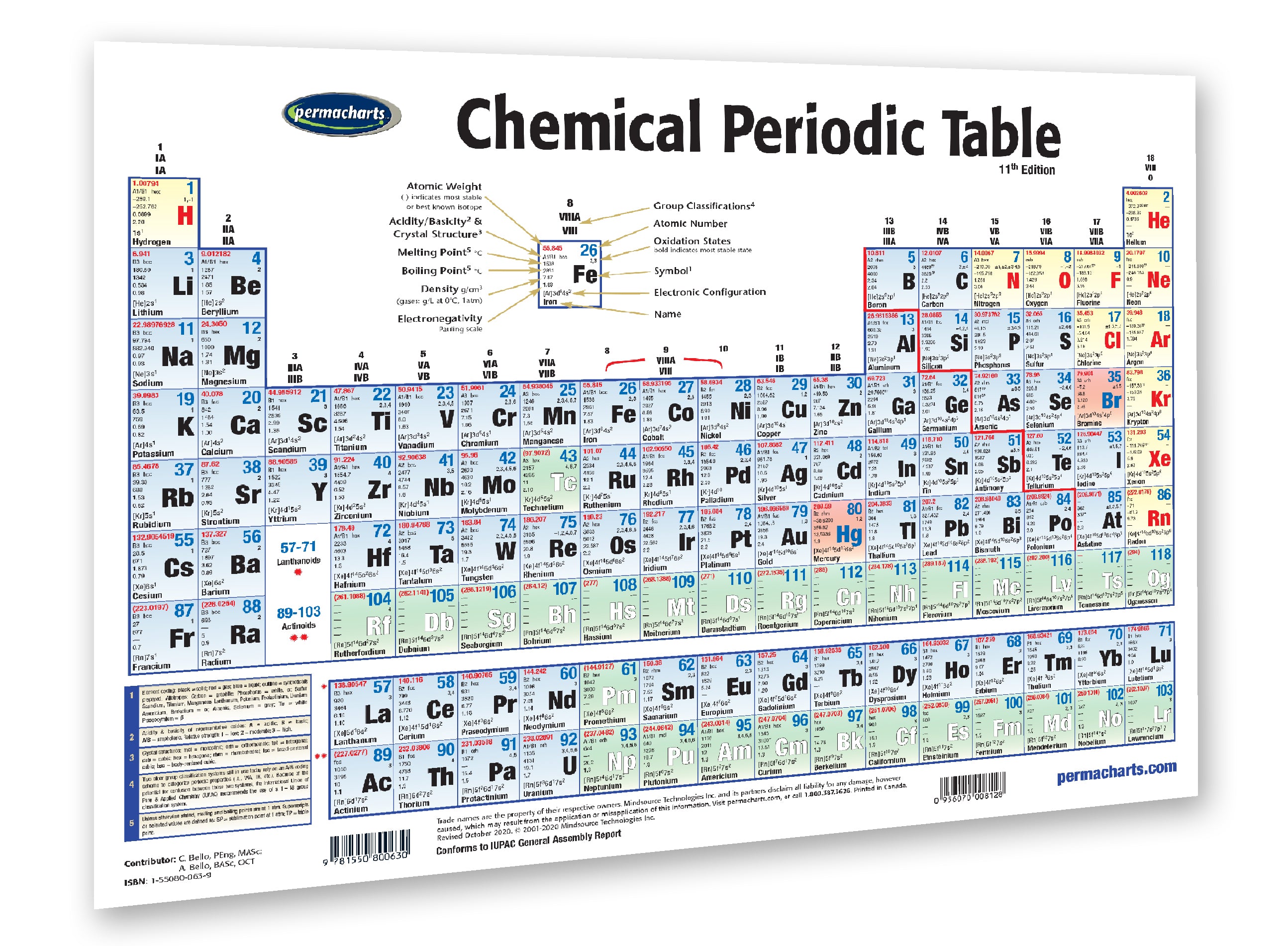
This would mean that indium’s atomic mass was actually 113, placing the element between two other metals, cadmium, and tin.\) The Periodic Table Showing the Elements in Order of Increasing Z Because elemental indium is a silvery-white metal, however, Mendeleev postulated that the stoichiometry of its oxide was really In 2O 3 rather than InO. If this atomic mass were correct, then indium would have to be placed in the middle of the nonmetals, between arsenic (atomic mass 75) and selenium (atomic mass 78). The element gallium was discovered four years after the publication of Mendeleevs table, and its properties matched up remarkably well with eka-aluminum, fitting into the table exactly where he had predicted. The atomic mass of indium had originally been reported as 75.6, based on an assumed stoichiometry of InO for its oxide. Handouts can provide only necessary information during test taking. Hide unnecessary or grade-level inappropriate information.
#Periodic table chemistry sudafedrine pdf
He discovered, for example, that the atomic masses previously reported for beryllium, indium, and uranium were incorrect. The periodic table is an arrangement of the elements in order of increasing atomic number. Ptable's new, up-to-date periodic table PDF and wide periodic table PDF are layered so you can choose exactly what you want to print, and are the perfect companion to the periodic table classroom poster. When the chemical properties of an element suggested that it might have been assigned the wrong place in earlier tables, Mendeleev carefully reexamined its atomic mass. The observed properties of gallium and germanium matched those of eka-aluminum and eka-silicon so well that once they were discovered, Mendeleev’s periodic table rapidly gained acceptance. Two of the blanks Mendeleev had left in his original table were below aluminum and silicon, awaiting the discovery of two as-yet-unknown elements, eka-aluminum and eka-silicon (from the Sanskrit eka, meaning “one,” as in “one beyond aluminum”).


 0 kommentar(er)
0 kommentar(er)
